For U.S. users: The VLVbio K18 assays have not been approved by the U.S. Food and Drug Administration
M30 Apoptosense® ELISA
Product no. 10011
The M30 Apoptosense® ELISA measures the amount of caspase cleaved keratin 18 in human serum and cell culture supernatants, as well as cell spheroids and xenograft models, reflecting the amount of apoptosis. The assays are based on the unique M30® antibody, which recognizes a neo-epitope of keratin 18 formed after caspase cleavage.
The M30 Apoptosense® ELISA is CE marked as a medical device for in vitro diagnostic use. All reagents are provided in a convenient ready-to-use format. The product is not cleared by the FDA for clinical use in the US.
M30 APOPTOSENSE® ELISA M30 APOPTOSENSE® ELISA M30 APOPTOSENSE® ELISA M30 APOPTOSENSE® ELISA M30 APOPTOSENSE® ELISA
Product characteristics
The test has 96 determinations: 7 standards, 2 controls and 39 test samples in duplicate.
The test has 96 determinations: 7 standards, 2 controls and 39 test samples in duplicate.
Assay procedure takes approximately 5 hours.
Sample type: human serum. Multiple freeze-thaw cycles of samples are well tolerated.
Reagent storage: +2 – 8 °C.
Can be split up for use at several occasions.

Assay Procedure
Assay Procedure of M30 Apoptosense® ELISA
Clinical IVD application for the M30 Apoptosense® ELISA:
Clinical IVD application
In Non-Alcoholic Fatty Liver Disease
As an aid in diagnosing Non-Alcoholic Steatohepatitis (NASH) in Non-Alcoholic Liver Disease suspect patients.
The test has 96 determinations: 7 standards, 2 controls and 39 test samples in duplicate.
As an intervention follow-up to check for treatment effect in NASH patients.
In Alcoholic Liver Disease
As an aid in diagnosing Alcoholic Steatohepatitis (ASH) in Alcoholic Liver Disease (ALD) suspect patients.
As a prognostic tool and treatment response prediction in ASH patients.
Research Fields
Hepatology
The M30 Apoptosense® ELISA measures the amount hepatocyte apoptosis in patients with liver diseases and is used to diagnose and screen for liver diseases such as Non-alcoholic Steatohepatitis, Alcoholic Steatohepatitis and Hepatitis B.
Oncology
The M30 Apoptosense® ELISA can specifically detect compounds that induce apoptosis, a major goal of anti-cancer drugs, as well as monitor the efficacy of therapy during the course of treatment.
Toxicology
Many pharmaceutical agents and substances are known to be hepatotoxic when administered in high doses, a common reason for patient hospitalization and withdrawal of approved drugs from the market. The M30 Apoptosense® ELISA can be used to detect elevated levels of ccK18 as a result of hepatotoxicity due to e.g. Drug-Induced Liver Injury or Toxicant-Associated Steatohepatitis.
M30 Apoptosense® ELISA in:

CLINICAL APPLICATION
Non-Alcoholic Steatohepatitis
NASH is a progressive and serious form of Non-alcoholic Fatty Liver Disease (NAFLD), and has during the last couple of decades become one of the most common causes of liver disease globally and a public health problem.
The number of affected patients is growing rapidly and the disease affects a considerable share of today’s global population.
CLINICAL APPLICATION
Alcoholic Steatohepatitis
Excessive alcohol use is a large global problem where around 300 million people around the world have an alcohol use disorder. This can lead to alcohol associated liver disease (ALD), a chronic liver disease that can further lead to Alcoholic Steatohepatitis (ASH), liver fibrosis and eventually cirrhosis.
Routine liver function tests used today often fail to detect and monitor liver damage due to ALD.
Together with other products
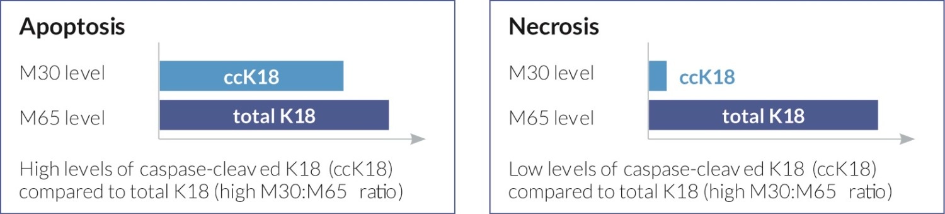
The assay can be combined with the M65® ELISA for further analysis of the cell death mode (necrosis or apoptosis). As both assays are calibrated against the identical reference, the combination of the M30 Apoptosense® ELISA and the M65® ELISA allows determination of the relative contribution of apoptosis to total cell death.