
11 December 2025
Thank you for being part of our 2025
Dear colleagues, partners, and friends, As we conclude another remarkable year, we want to extend our sincere gratitude to each of you; our distributors, collaborators, and partners around the world.2024–2025 has been a year defined by progress, dialogue, and renewed momentum in the MASLD/MASH field. We are truly grateful for the continued trust, engaged discussions, […]
Read more12 June 2025
Global Fatty Liver Day
1 in 3 adults has fatty liver disease. And many don’t even know it. Today on Global Fatty Liver Day, we stand with the global health community to raise awareness of this silent epidemic and the power of early detection.At VLVbio, we’re committed to supporting healthcare professionals with diagnostic tools that enable timely action. Read […]
Read more
22 May 2025
Play For Your Liver
Liver disease isn’t just an adult problem. Today, 1 in 10 children are at risk of MASLD (Metabolic dysfunction-associated steatotic liver disease), and the numbers are rising fast. At VLVbio, we believe that prevention starts young – through movement, healthy habits, and community support. That’s why we’re proud to sponsor a local Swedish youth football […]
Read more
25 April 2025
The EASL Congress 2025
Meet our team in Amsterdam and let’s discuss M30® as a NIT in MASH, AH and DILI!
Read more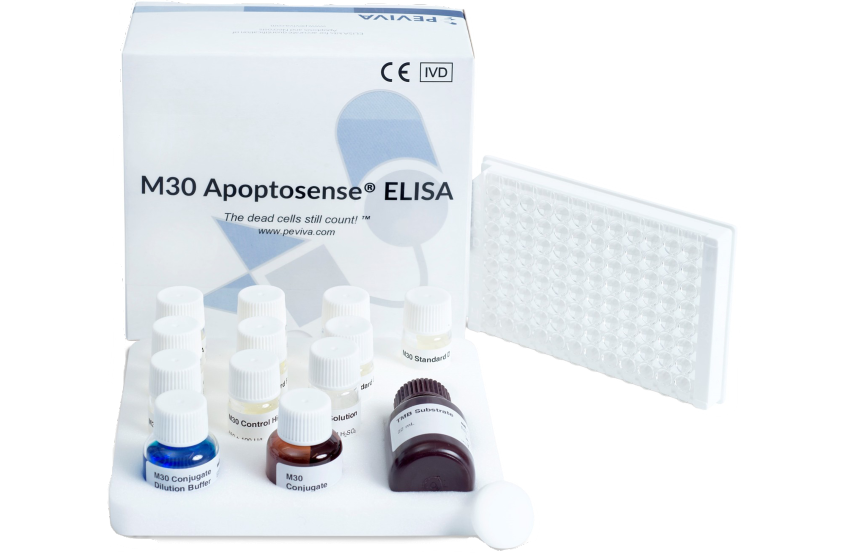
M30 Apoptosense® in NASH
The M30 Apoptosense® ELISA can be used as an aid in diagnosing patients with NASH which would allow for an early identification of patients at risk of developing late stage liver disease and would benefit from specialist care.
Diagnosing NASH also allows an earlier intervention prior to the onset of fibrosis and severe liver injury.
NAFLD NASH ALD ASH DILI ALF ACLF NAFLD NASH ALD ASH DILI ALF ACLF NAFLD NASH ALD ASH DILI ALF ACLF NAFLD NASH ALD ASH DILI ALF ACLF NAFLD NASH ALD ASH DILI ALF ACLF

NAFLD – a global disease
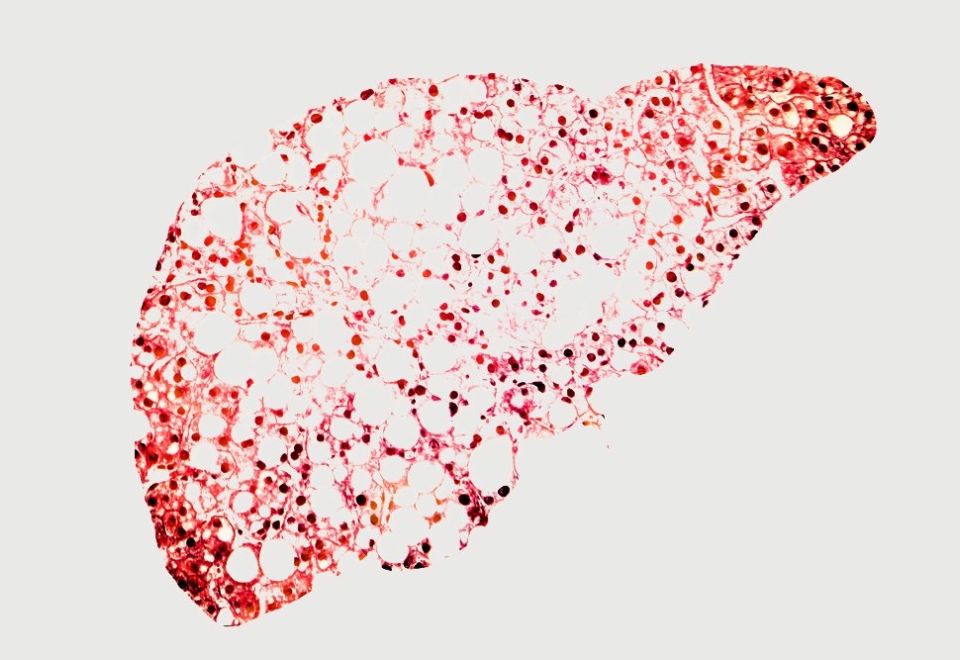
Non-alcoholic fatty liver disease (NAFLD), a condition where fat accumulates in the liver without the excess consumption of alcohol, has become the most common cause of liver disease in the Western world, and is quickly rising to become the primary cause of liver transplants.
Due to the rise in obesity and diabetes, NAFLD is estimated to affect 30% of the global population, with a high prevalence on all continents.
NASH
NAFLD starts out as steatosis which is an accumulation of fat in the liver and may further progress to non-alcoholic steatohepatitis (NASH), a more serious form of NAFLD, where the liver has become inflamed.
NASH is a potentially fatal condition that affects 5% of the global adult population and can further develop into creation of fibrotic tissue in the liver, with possible cirrhosis as a result.
The right to good health is a fundamental human right. Therefore we strive to offer and facilitate high-quality biomarker assays to detect and measure liver disease in an effective, sensitive and cost-effective way.

Long Experience
Based on antibody technology invented more than 20 years ago at the Karolinska Institute in Sweden, VLVbio has a deep and profound experience within epithelial cell death in different application fields.
Cost-effective and easy to use
Our M30 Apoptosense® ELISA is a non invasive, cost-effective tool for the aid in diagnosing NASH. The M30® can with adequate sensitivity and specificity aid in early detection of NAFLD patients while the disease is still reversible. Our products are easily available globally!
Clinical Validation
In order to validate and pin-point the adequate diagnostic solution in NAFLD healthcare, we are conducting several clinical trials worldwide to evaluate the use of M30 Apoptosense® in NASH diagnosis.
Clinical pharmacology trials
While there are still no FDA or EMA approved drugs for NASH, there are many trials ongoing in different phases to find a pharmaceutical treatment. Our products are used in over 60 active NAFLD trials as a screening tool and endpoint measurement for treatment effect.
Research Collaborations
As part of our mission to aid in finding and validating the best biomarkers to advance towards diagnosis and monitoring of NASH, we are collaborating with large joint governmental collaborations with leading clinical academic centers, regulatory bodies, and the industry. We are currently part of the LITMUS and NAIL-NIT consortiums.
Continuous Innovation
We are committed to satisfying customer requirements for quality, reliability, and support for all of our products. We are dedicated to continuous innovation and improvement in all aspects of our business, constantly looking to improve our products as well as expand our product portfolio.








17 April 2024
Home Slider
K18 – the Key to Unlocking Informed Decisions in MASH Drug Trials
Join us for an enlightening webinar presented by Jessica Tuohy, a distinguished partner and colleague from the US, focusing on the significance of K18 as a biomarker in the development of treatments for MASH (Metabolic Associated Steatohepatitis). This webinar is essential for drug developers, doctors, researchers, and key opinion leaders in the field of metabolic liver diseases, offering deep insights and key research findings.
Event Details:
- Date: Tuesday, April 23
- Time: [1pm EDT, 7pm CET]
Webinar Overview: This session will cover several critical areas:
- – An Introduction to the Growing Epidemic of MASLD (Metabolic Associated Steatotic Liver Disease)
- – Challenges in Developing Therapies for the Treatment of MASH
- – An Overview of K18 and Its Role in MASH Pathogenesis
- – A Review of Data That Support the Value of K18 as a Biomarker for Assessing the Efficacy of Drug Candidates in MASH Clinical Trials
Who Should Attend:
- – Drug developers in the field of MASLD and MASH.
- – Physicians and healthcare professionals specializing in hepatology, gastroenterology, and related fields.
- – Medical researchers focusing on liver disease and drug development.
- – Key opinion leaders in metabolic liver diseases.
Registration: Please register here to secure your spot in this informative webinar. Registration is required to access this online event.
Additional Information: If you are unable to attend the live event, a recording will be made available to all registered participants, ensuring you can access this webinar at your convenience.
We look forward to welcoming you to what promises to be an informative and transformative session.
For more information, please contact us at marketing@vlvbio.com.
Don’t miss out on the chance to advance your knowledge in MASH treatment and diagnostics. Reserve your spot today!
23 January 2024
Blog
Launch of IMMUNIS Cytokeratin 18F EIA by Institute of Immunology, Japan
We are thrilled to share a momentous achievement in our longstanding collaboration with our esteemed Japanese partners, the Institute of Immunology. This announcement comes during a meeting filled with collaboration and shared successes as Mr. Ito, Mr. Akira, and Ms. Yukiko from the Institute of Immunology paid a visit to our headquarters in Stockholm.
During this collaborative visit, we had the pleasure of discussing plans for the imminent launch of the IMMUNIS Cytokeratin 18F EIA, a project that holds significant promise in the realm of diagnostic medicine. This project has been a work of dedication and innovation, and the collaboration between VLVbio and the Institute of Immunology has been instrumental in its success!
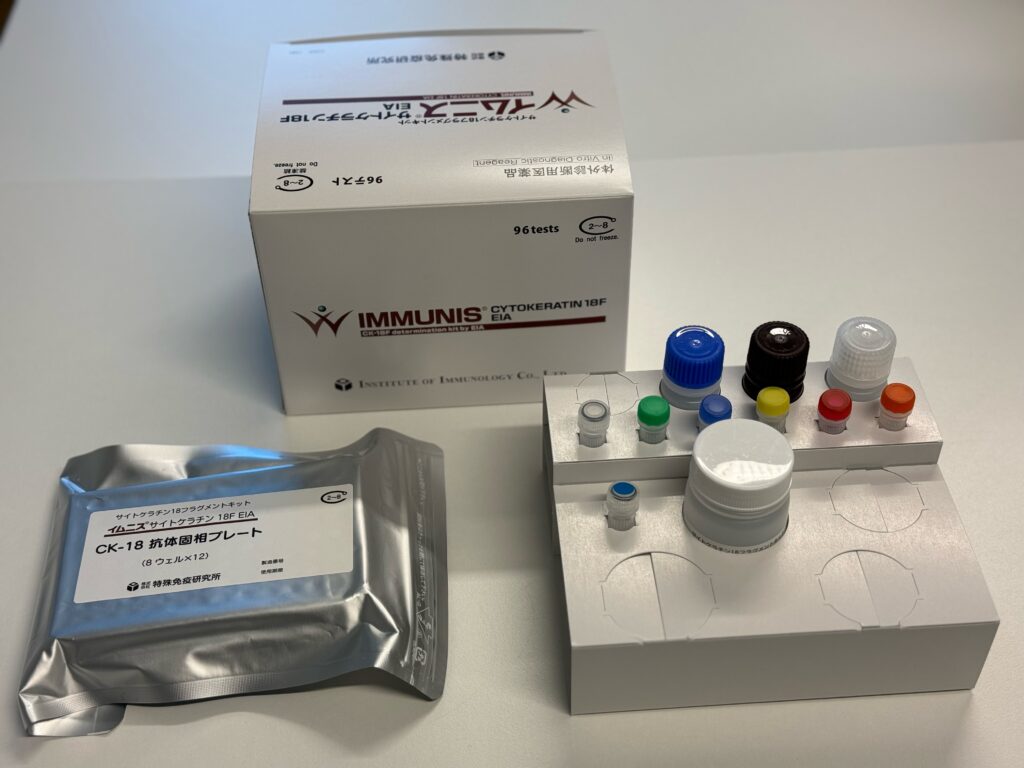
We are happy that the IMMUNIS Cytokeratin 18F EIA has gained official approval as an In Vitro Diagnostic (IVD) for the diagnosis of Non-Alcoholic Steatohepatitis (NASH) from the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan! This recognition underscores the product’s efficacy and reliability in contributing to the diagnosis and management of NASH, a condition of growing concern globaly as the epidemiology of the disease keeps increasing at an alarming rate. The need for a biomarker to identify patients with NASH and who are at risk of significant liver disease is of utmost importance, and we are happy that the Keratin 18 assay can assist in that!
The IMMUNIS Cytokeratin 18F EIA ELISA kit
Of equal importance, the IMMUNIS Cytokeratin 18F EIA, developed by the Institute of Immunology, has been included in the national health insurance system in Japan. This recognition ensures widespread accessibility, facilitating broader usage of this diagnostic tool within the Japanese healthcare community.
We are pleased to announce that the official launch date for the IMMUNIS Cytokeratin 18F EIA is scheduled for February 26th to the Japanese market. This date signifies the conclusion of a meticulous research and development process and exemplifies the collaborative efforts between VLVbio and our esteemed partners at the Institute of Immunology. The IMMUNIS Cytokeratin 18F EIA, launched by the Institute of Immunology, is poised to make a substantial impact on the field of NASH diagnosis, providing healthcare professionals with an additional tool to enhance their diagnostic capabilities in Japan.
As we celebrate this milestone and the successful visit from our partners, we extend our gratitude to all individuals who have contributed to the realization of this project, including the dedicated team members and esteemed partners at the Institute of Immunology. Congratulations to you all!

Institute of Immunology president Mr. Ito Yukio and VLVbio CEO
Mrs. Slavica Brnjic holding the finished IMMUNIS Cytokeratin 18F assay.
11 December 2025
Home Slider
Thank you for being part of our 2025
Dear colleagues, partners, and friends,
As we conclude another remarkable year, we want to extend our sincere gratitude to each of you; our distributors, collaborators, and partners around the world.
2024–2025 has been a year defined by progress, dialogue, and renewed momentum in the MASLD/MASH field. We are truly grateful for the continued trust, engaged discussions, new scientific contacts, and the many constructive conversations we’ve shared with you throughout the year.
The global MASLD landscape is evolving faster than ever. The release of Rezdiffra™ in the United States has already brought meaningful benefit to many patients, marking a long-awaited step forward in treatment availability. With the recent approval in Europe, we are excited to see this progress finally reach European patients, which is a milestone the field has been anticipating for decades.
Likewise, the approval of semaglutide for MASH adds an important therapeutic option. After years of limited tools, we are now entering a new phase where patients with MASH can finally receive the help they need.
But as treatment availability expands, a new challenge has emerged:
How do we effectively monitor patients on therapy?
This question has been raised repeatedly in our conversations with clinicians, KOLs, and partners this year, and it is becoming one of the central needs in clinical practice.
In this evolving landscape, M30 Apoptosense® ELISA has shown clear potential to fill an important part of this gap.
Evidence from multiple clinical trials, including Madrigal’s phase III MAESTRO-NASH trial, has shown that M30® (ccK18) is one of the leading blood-based indicators of treatment response, outperforming several structural NITs in early prediction of change. Importantly, M30® offers:
- – Early, biology-based insight into hepatocellular injury
- – Reliable tracking of treatment effect before structural changes become visible
- – A practical solution for clinics without access to advanced imaging such as MRI-PDFF
- – A low-cost, accessible assay that integrates easily into routine follow-up workflows
As more patients in the US and Europe begin therapy, the need for simple, repeatable, blood-based monitoring tools has never been clearer. We are proud that our M30 Apoptosense® ELISA continues to serve as a scientifically grounded, widely studied option to support clinicians and improve real-world treatment follow-up.
Looking ahead to 2026, we are excited to continue supporting you; through scientific collaboration, distributor partnerships, educational materials, and tools that strengthen your local markets. Your work is essential for bringing knowledge, clarity, and better care to patients worldwide, and we value the role you play in this shared mission.
From all of us at VLVbio:
Thank you for a productive, inspiring, and forward-moving year.
We look forward to continuing our work in 2026.
The VLVbio Team

12 June 2025
Home Slider
Global Fatty Liver Day
1 in 3 adults has fatty liver disease. And many don’t even know it.
Today on Global Fatty Liver Day, we stand with the global health community to raise awareness of this silent epidemic and the power of early detection.
At VLVbio, we’re committed to supporting healthcare professionals with diagnostic tools that enable timely action. Read more on how the VLVbio products assist diagnosing steatotic liver diseases here!
Know it. Test it. Treat it. 💚
22 May 2025
Home Slider
Play For Your Liver
Liver disease isn’t just an adult problem.
Today, 1 in 10 children are at risk of MASLD (Metabolic dysfunction-associated steatotic liver disease), and the numbers are rising fast.
At VLVbio, we believe that prevention starts young – through movement, healthy habits, and community support.
That’s why we’re proud to sponsor a local Swedish youth football team, promoting an active lifestyle and early liver health awareness.
Because every goal scored is a step toward a healthier future! ⚽
Watch our short campaign video below to see how we’re helping raise awareness and protect the next generation, one active child at a time.
#PlayForYourLiver
25 April 2025
Home Slider
The EASL Congress 2025
We are heading to Amsterdam for the EASL Congress together with the European Association for the Study of the Liver!
We’re happy to yet again be exhibiting at #EASLcongress with EASL | The Home of Hepatology! We are happy to yet again be an industry partner for the biggest liver event in Europe!
The latest in hepatology is happening 📅 7–9 May.
🤝 Join us at the highlight of the year! Register today, and lets meet and discuss liver biomarkers at our booth.
19 March 2025
Blog
High-Risk Populations and Targeted Interventions in MASLD
Metabolic Associated Steatotic Liver Disease (MASLD) affects millions worldwide, but certain populations face significantly higher risks due to underlying metabolic conditions and lifestyle factors. Early identification and tailored interventions for these high-risk groups are critical in mitigating disease progression and improving outcomes.
In this post, we focus on identifying these populations and the role of personalized diagnostics and care strategies.
Who Is at Risk?
MASLD is closely linked to metabolic dysfunction, making individuals with certain conditions particularly vulnerable to the disease. Recognizing these high-risk groups allows healthcare providers to focus resources and interventions where they are needed most.
1. Individuals with Type 2 Diabetes
Research indicates that up to 70% of individuals with Type 2 diabetes also have MASLD, often progressing silently toward fibrosis or cirrhosis. The interplay between insulin resistance and liver fat accumulation accelerates liver damage in this group.
2. Patients with Obesity
Obesity is one of the strongest predictors of MASLD, affecting up to 90% of this population. Excess visceral fat contributes to liver fat deposition and inflammation, increasing the likelihood of progression to more severe disease stages.
3. Additional High-Risk Groups
· Individuals with Dyslipidemia: Elevated triglycerides and low HDL cholesterol are common in MASLD patients.
· Patients with Hypertension: Often part of metabolic syndrome, hypertension contributes to the overall disease burden.
· Family History of Liver Disease: Genetic predisposition can heighten MASLD risk.
Identifying these populations during routine care, such as diabetes or obesity management, provides an opportunity to diagnose MASLD early, even before symptoms appear.
The Importance of Tailored Diagnostics
For high-risk populations, non-invasive diagnostic tools are invaluable in stratifying patients by their risk of progression and guiding care decisions.
M30 Apoptosense® ELISA
Biomarkers like M30 Apoptosense® ELISA measure hepatocyte apoptosis, offering a non-invasive way to detect early liver injury. In high-risk groups, M30 can be used to:
· Identify patients likely to progress to advanced disease stages.
· Monitor the effectiveness of lifestyle or medical interventions.
· Provide a measurable outcome to guide therapeutic decisions.
The Benefits of Tailored Diagnostics
· Risk Stratification: Directs resources toward patients at highest risk, avoiding unnecessary testing for low-risk individuals.
· Proactive Monitoring: Allows clinicians to track changes over time and intervene when needed.
· Cost-Effectiveness: Reduces healthcare expenditures by preventing advanced-stage complications.
By focusing on non-invasive, scalable tools, healthcare providers can effectively manage MASLD in high-risk populations without relying on resource-intensive methods like biopsies.
Targeted Interventions for High-Risk Groups
Once high-risk patients are identified, personalized interventions can significantly improve outcomes and prevent progression to severe disease stages.
1. Lifestyle Modifications
Lifestyle changes remain the cornerstone of MASLD management. Evidence shows that:
· Losing 7–10% of body weight can reduce liver fat, inflammation, and fibrosis.
· Adopting a Mediterranean diet can improve liver health by emphasizing whole foods, healthy fats, and reduced sugar intake.
· Structured exercise programs improve metabolic function and reduce liver fat.
2. Pharmacological Treatments
Emerging therapies targeting specific mechanisms of MASLD, such as liver fat accumulation or fibrosis, are becoming available. These treatments complement lifestyle changes in patients who require additional intervention.
3. Regular Monitoring
Tools like M30 Apoptosense® ELISA enable healthcare providers to track the impact of interventions and make timely adjustments. Regular follow-ups also reassure patients of their progress, encouraging long-term adherence.
Preventing the Burden of Advanced Liver Disease
High-risk populations are at an increased likelihood of progressing to cirrhosis, liver failure, or hepatocellular carcinoma. The long-term economic and clinical burden of these outcomes is substantial. By focusing on early diagnosis and targeted interventions:
· The progression to severe disease can be significantly reduced.
· Patient quality of life improves.
· Healthcare systems can avoid the high costs associated with advanced-stage treatments.
Conclusion
Targeting high-risk populations with tailored diagnostics and interventions is a critical step in addressing the global burden of MASLD. Non-invasive tools like M30 Apoptosense® ELISA, combined with lifestyle changes and emerging therapies, provide healthcare providers with the resources needed to deliver proactive, patient-centered care.
As MASLD continues to rise worldwide, adopting this targeted approach will play a pivotal role in improving outcomes and reducing the disease’s impact on patients and healthcare systems. Read more here!
11 December 2024
Blog
Managing MASLD: Lifestyle Changes and Tools for Long-Term Liver Health
Metabolic Associated Steatotic Liver Disease (MASLD) is a growing global health concern, with many patients unaware of their condition until significant liver damage has occurred. Early diagnosis is critical, but what happens after a patient is diagnosed? The next step is effective management to prevent disease progression and improve liver health outcomes.
In this post, we explore how lifestyle interventions and diagnostic tools can help patients manage MASLD and how healthcare providers can play a pivotal role in long-term care.
The Role of Lifestyle Changes in MASLD Management
Lifestyle interventions are currently the cornerstone of MASLD management. Studies show that losing just 7–10% of body weight can significantly improve liver health by reducing liver fat, inflammation, and fibrosis (Vilar-Gomez et al., Hepatology, 2015). Patients are often advised to:
- Adopt a Healthy Diet:
Diets like the Mediterranean diet, which emphasize fruits, vegetables, whole grains, and healthy fats, have been shown to improve liver function. Reducing sugar and saturated fat intake is also crucial. - Increase Physical Activity:
Regular exercise, including aerobic and resistance training, can reduce liver fat and improve metabolic health. - Maintain Consistency:
Adherence to these changes is key. Providing patients with tailored recommendations and ongoing support can make a significant difference in sustaining these changes.
Healthcare providers play a crucial role in educating patients about these interventions and monitoring their progress over time.
The Importance of Monitoring Tools
In managing MASLD, effective monitoring is essential for assessing treatment efficacy, tracking disease progression, and preventing advanced liver conditions such as cirrhosis. While lifestyle changes are central to improving liver health, non-invasive tools provide valuable insights that guide clinical decision-making and optimize patient outcomes.
The Role of Serum Biomarkers
Among the available tools for MASLD monitoring, serum biomarkers have gained significant attention due to their ability to detect ongoing liver injury and apoptosis. The M30 Apoptosense® ELISA (M30®), which measures caspase-cleaved keratin18 (ccK18), provides a window into hepatocyte apoptosis, an early indicator of liver damage. Research has demonstrated its utility in diverse clinical scenarios:
In a randomized trial, M30® was used to evaluate the impact of weight loss through liraglutide treatment and lifestyle modifications in patients with MASLD. The study showed significant reductions in M30® levels, reflecting improvements in liver injury alongside reductions in body weight and liver fat (Khoo et al., Liver International, 2019).
Following bariatric surgery, M30® identified ongoing liver injury in some patients, even as weight loss improved overall metabolic health. This insight proved valuable for tailoring follow-up care (Hempel et al., Journal of Clinical Medicine, 2021)
By detecting apoptotic activity, M30® provides clinicians with a reliable tool to monitor treatment progress and refine therapeutic strategies.
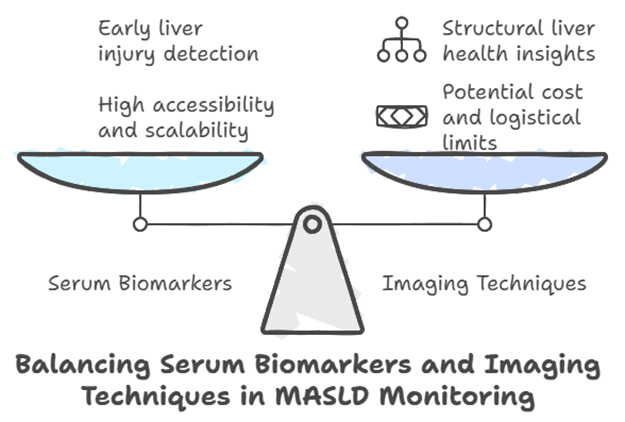
Complementary Imaging Techniques
In addition to serum biomarkers, imaging techniques such as transient elastography and MRI-based modalities play an important role in assessing liver fat and fibrosis. These tools complement biomarkers by providing structural insights into liver health, allowing for a more comprehensive evaluation of MASLD progression.
While imaging tools are effective, their availability may be limited in some settings due to cost or logistical challenges. This underscores the value of biomarkers like M30®, which are accessible and scalable for routine monitoring.
Integrating Monitoring into MASLD Care
The integration of non-invasive tools into MASLD management offers several benefits:
Enhancing Patient Engagement: Demonstrating objective improvements in liver health can motivate patients to adhere to therapeutic regimens and lifestyle adjustments.
Identifying Non-Responders: Regular monitoring allows clinicians to identify patients who may not be responding to interventions, enabling timely adjustments to care plans.
Tracking Long-Term Progress: Tools like M30® provide measurable feedback on the impact of lifestyle changes and pharmacological treatments over time.
A Balanced Approach to MASLD Monitoring
Effective MASLD management requires a combination of approaches, including biomarkers, imaging, and clinical assessments. The M30 Apoptosense® ELISA is a valuable component of this toolkit, offering an accessible method for assessing liver health and tracking treatment outcomes. Paired with imaging and clinical evaluations, M30® can help healthcare providers develop personalized and proactive care plans for their patients.
Preventing Progression to Advanced Liver Disease
Effective MASLD management can prevent progression to advanced conditions like cirrhosis or hepatocellular carcinoma (HCC). Regular follow-up using non-invasive tools allows healthcare providers to:
- – Identify patients at risk of progression early.
- – Adjust treatment plans based on real-time data.
- – Reassure patients when improvements are seen, encouraging adherence to lifestyle changes.
Studies suggest that consistent monitoring and management can significantly reduce the incidence of severe liver outcomes, improving both patient quality of life and healthcare cost efficiency (Younossi et al., Journal of Hepatology, 2020).
A Collaborative Approach to MASLD Care
Managing MASLD requires a collaborative approach between healthcare providers and patients. Shared decision-making, clear communication, and personalized care plans are essential to ensure long-term success. Non-invasive tools like serum biomarkers and imaging are valuable assets in this process, helping both clinicians and patients stay informed about disease progression and treatment outcomes.
Conclusion
MASLD management doesn’t end with diagnosis. Lifestyle changes and ongoing monitoring are critical to improving liver health and preventing severe complications. By incorporating non-invasive tools like M30 Apoptosense® ELISA into routine follow-up care, healthcare providers can better track treatment effectiveness and support patients on their journey to better health.
As MASLD prevalence continues to rise, combining lifestyle interventions with advanced diagnostic tools is essential to addressing this global health challenge. VLVbio is proud to support this shift toward earlier, more accurate detection through our non-invasive testing solutions. Read more here!
15 November 2024
Blog
The Importance of Non-Invasive Testing in Early MASLD Diagnosis
As part of our ongoing effort to raise awareness about Metabolic Associated Steatotic Liver Disease (MASLD), we are exploring how advances in non-invasive testing (NITs) can transform early diagnosis. Early detection is crucial in preventing MASLD from progressing to advanced stages like fibrosis or cirrhosis, where treatment becomes more complex. NITs, such as serum biomarkers, imaging technologies, and elastography, are helping healthcare providers catch the disease before significant liver damage occurs.
Why Early Diagnosis Matters
MASLD often progresses without symptoms in its early stages, meaning patients can unknowingly live with the disease for years. By the time symptoms appear—often in the form of fatigue, abdominal swelling, or jaundice—patients may already have advanced fibrosis or cirrhosis. Early diagnosis is essential to allow healthcare providers to implement lifestyle interventions and treatment strategies that can slow or even reverse the disease.
Overview of Non-Invasive Testing Options
Traditionally, liver biopsies were the primary method for diagnosing liver diseases like MASLD. While still valuable in some cases, biopsies are invasive and may carry risks. The field has since evolved, and there are now several non-invasive methods available to assess liver health.
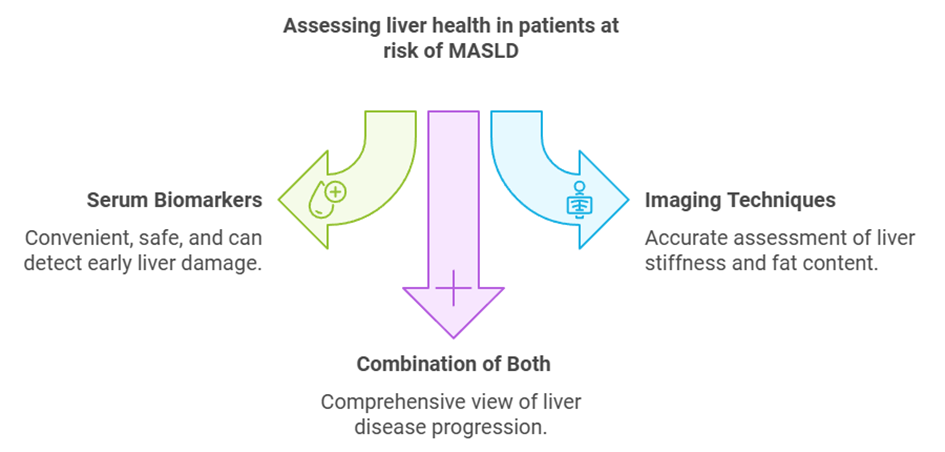
1. Serum Biomarkers
Serum biomarkers are among the most promising tools for detecting early liver disease. These tests measure specific proteins in the blood that are associated with liver damage, such as cell death (apoptosis) and fibrosis. Biomarkers, like the M30 Apoptosense® ELISA, help detect liver cell apoptosis, an early sign of disease progression. These tests are convenient, safe, and can be easily integrated into routine care for high-risk patients.
2. Imaging Techniques
Several imaging technologies are available to assess liver stiffness and fat content, helping clinicians evaluate liver health without invasive procedures. Elastography is commonly used to measure liver stiffness, while other imaging techniques like MRI-PDFF can quantify liver fat. These methods have significantly improved the accuracy of early diagnosis, although they may not always capture the underlying biochemical changes that serum biomarkers detect.
Both biomarkers and imaging tools offer valuable insights into liver health, and each has its place depending on the patient’s condition and risk factors. When used together, these technologies can provide a comprehensive view of liver disease progression.
How Non-Invasive Tests Improve Patient Outcomes
By utilizing non-invasive tests, healthcare providers can screen high-risk populations—such as individuals with obesity, Type 2 diabetes, or metabolic syndrome—more frequently and with less discomfort to the patient. This allows for:
Ongoing Monitoring: NITs allow clinicians to monitor disease progression and treatment effectiveness over time, adjusting interventions as needed.
Earlier Interventions: Once MASLD is diagnosed, patients can begin lifestyle changes (such as diet and exercise) or medical treatment earlier, improving the likelihood of reversing or halting the disease.
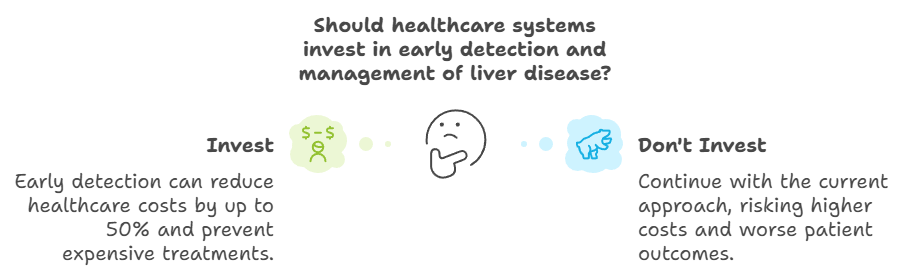
Cost-Effectiveness of Early Diagnosis
Early detection not only improves patient outcomes but also offers significant cost savings for healthcare systems. A study found that managing liver disease at earlier stages can reduce healthcare costs by up to 50% compared to treating advanced-stage complications such as cirrhosis or liver cancer (Younossi et al., Journal of Hepatology, 2020). In the U.S., the annual healthcare costs related to MASLD are estimated to exceed $100 billion, a number that continues to rise as MASLD prevalence increases (Younossi et al., Hepatology, 2020).
Research also highlights that patients with cirrhosis incur nearly 4.5 times higher medical costs than those diagnosed early. For example, annual direct medical costs for patients with cirrhosis are approximately $35,000, compared to $7,800 for those diagnosed at earlier stages (Tapper et al., Hepatology, 2020; Younossi et al., Journal of Hepatology, 2020). Early diagnosis can therefore prevent the need for expensive treatments such as hospitalizations and liver transplants, significantly reducing the economic burden on healthcare systems.
Conclusion
Non-invasive testing has opened new doors in the early diagnosis and management of MASLD. From serum biomarkers to imaging techniques, these tools are making it easier for healthcare professionals to diagnose liver disease before it progresses. By using these advanced diagnostics, clinicians can help prevent severe outcomes and improve patient quality of life. VLVbio is proud to support this shift toward earlier, more accurate detection through our non-invasive testing solutions. Read more here!
17 October 2024
Blog
Understanding MASLD and Its Growing Global Prevalence
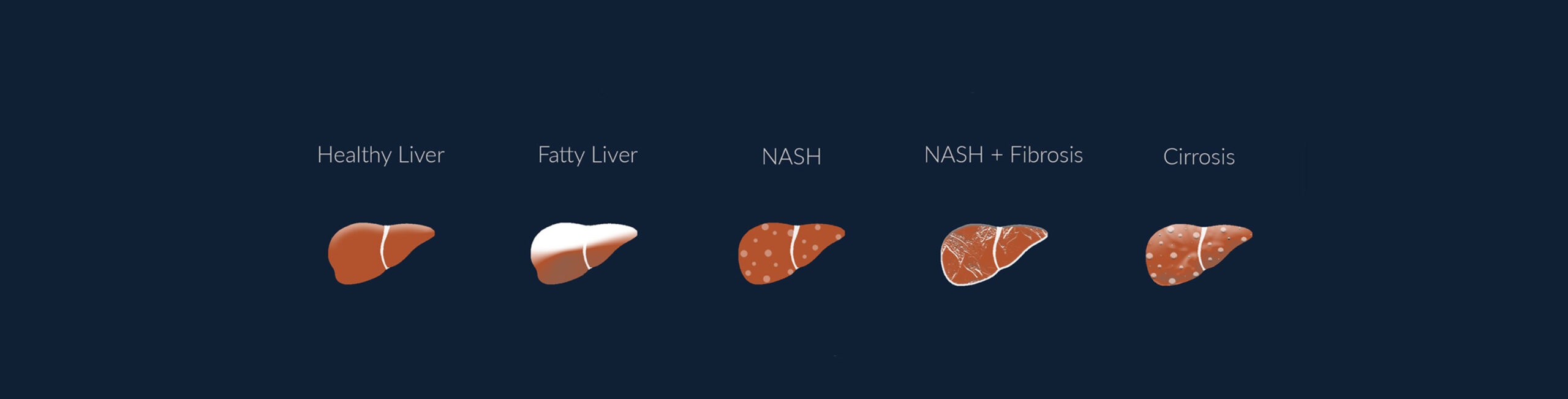
Metabolic Associated Steatotic Liver Disease (MASLD), also known as Non-Alcoholic Fatty Liver Disease (NAFLD), has emerged as a major global health challenge. Affecting roughly 30% of the world’s population, MASLD is closely tied to the rising rates of obesity, Type 2 diabetes, and metabolic syndrome. This condition, which can progress from simple liver fat accumulation to severe stages like MASH, fibrosis or cirrhosis, requires urgent attention from healthcare professionals to manage its growing prevalence.
What is MASLD?
MASLD is a spectrum of liver diseases where excess fat builds up in the liver without significant alcohol consumption. The condition ranges from simple steatosis (fatty liver) to more severe forms like metabolic dysfunction-associated steatohepatitis (MASH), liver fibrosis, and cirrhosis. The key risk factors driving MASLD are metabolic conditions such as obesity and insulin resistance.
The Growing Global Prevalence of MASLD
Over the past few decades, the prevalence of MASLD has steadily increased across the globe. Recent studies reveal that 30% of the world’s population is affected by MASLD, a significant rise from the 25% reported in 2016. In certain regions like Latin America and the Middle East, the prevalence rates exceed 40%, driven by the increasing rates of obesity and metabolic syndrome. By 2030, the number of MASLD cases is expected to surge, particularly in Southeast Asia, North America, and Western Europe, as the global burden of metabolic disorders continues to rise (The Global Epidemic of Metabolic Fatty Liver Disease, 2024).
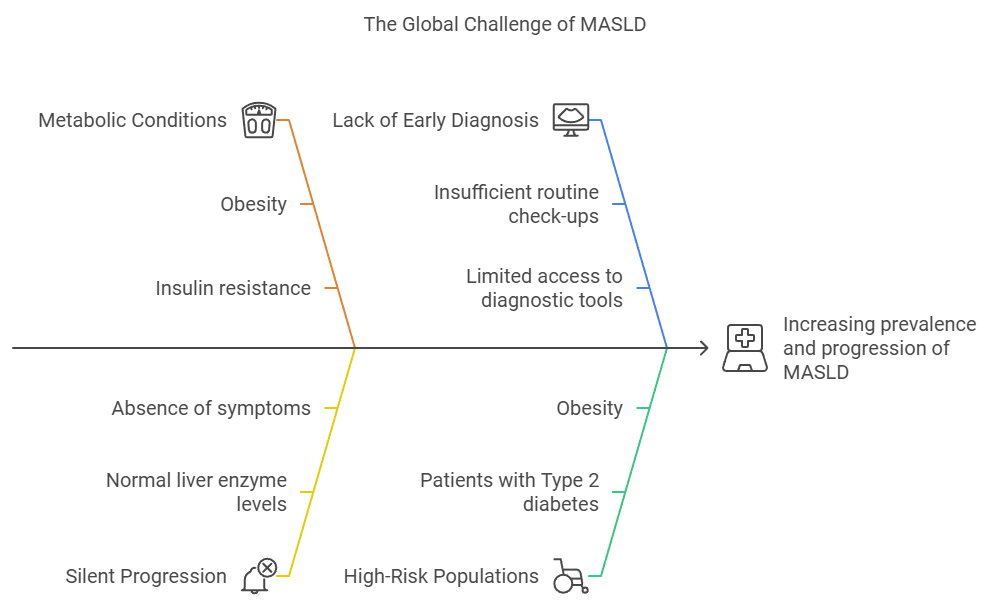
This growing burden makes early detection more critical than ever. The silent progression of MASLD, particularly among high-risk groups such as those with Type 2 diabetes or obesity, often leads to late-stage liver disease by the time it’s detected. Early screening and intervention can prevent end-stage complications like cirrhosis and liver failure.
Why Early Diagnosis is Critical
Early diagnosis of MASLD is pivotal in preventing the disease from progressing to more severe stages, such as fibrosis, cirrhosis, or hepatocellular carcinoma (HCC). MASLD often develops silently, with patients experiencing few or no symptoms in the early stages. As a result, the disease can go undetected until it advances to a point where treatment options are limited, and the risk of irreversible liver damage increases.
Silent Progression to Severe Disease
Many patients with MASLD are unaware of their condition until it has progressed significantly. This is because MASLD often presents with normal liver enzyme levels, which may give a false sense of security during routine checkups. Without early intervention, MASLD can progress to MASH, a more severe form that involves liver inflammation and fibrosis. From there, the disease can further develop into cirrhosis or even liver cancer. The longer the disease goes undiagnosed, the fewer options remain for successful intervention. Detecting MASLD early provides a critical window for lifestyle changes and medical management that can reverse or slow its progression.
“I had no idea my liver was deteriorating for years. I felt fine until one day, my legs started swelling, and I felt extreme fatigue. By then, it was too late — my doctor told me I had cirrhosis, and my liver was severely damaged.” – MASLD patient with cirrhosis
Preventing Cirrhosis and Liver Cancer
Early diagnosis allows healthcare providers to implement interventions that can prevent patients from progressing to the advanced stages of liver disease. Patients with MASLD who are diagnosed early have a higher likelihood of responding to non-invasive treatments such as weight loss, dietary changes, and glucose control. Studies have shown that even a modest weight loss of 7-10% of body weight can significantly improve liver histology and prevent disease progression. When MASLD is detected at the stage of simple steatosis, the risk of developing cirrhosis or liver cancer is much lower than if the disease is allowed to progress without intervention.
In advanced stages like cirrhosis, the liver’s ability to function is severely compromised, and patients may require liver transplantation. Moreover, cirrhosis significantly increases the risk of developing hepatocellular carcinoma, a leading cause of cancer-related deaths worldwide. Therefore, detecting MASLD in its early stages can significantly reduce the incidence of both cirrhosis and liver cancer, which are often associated with poor prognoses.
Role of Non-Invasive Diagnostics
Recent advancements in non-invasive diagnostic tools have made early detection of MASLD easier and more accessible for clinicians. Tools such as imaging and serum biomarkers allow healthcare providers to assess liver fat and fibrosis without the need for invasive liver biopsies. This is particularly valuable in primary care settings, where regular screening of high-risk populations (such as those with obesity, diabetes, or metabolic syndrome) can be incorporated into routine check-ups.
These diagnostic tools also allow for risk stratification, enabling clinicians to categorize patients based on their likelihood of progressing to advanced liver disease. This helps in tailoring interventions and deciding when to refer patients to specialists for more advanced care, reducing unnecessary referrals and allowing for more efficient healthcare resource allocation.
Managing High-Risk Populations
Patients with Type 2 diabetes, obesity, and other metabolic disorders are at particularly high risk for MASLD. In fact, up to 70% of people with Type 2 diabetes and 90% of obese individuals have MASLD. Early diagnosis in these populations is crucial, as they are more likely to progress to severe liver disease. By integrating liver health assessments into regular diabetes or obesity management plans, healthcare providers can catch MASLD before it progresses, allowing for earlier lifestyle modifications and potentially reducing the burden of advanced liver disease in these high-risk groups.
Improving Patient Outcomes and Reducing Costs
Early diagnosis and intervention not only improve patient outcomes but also reduce healthcare costs in the long run. Treating MASLD in its early stages is far less costly than managing advanced liver disease, which often requires hospitalizations, expensive treatments, or even liver transplantation. Moreover, patients diagnosed early are more likely to adhere to preventative measures such as diet and exercise, which can greatly improve liver health and reduce the need for long-term medical intervention.
Conclusion
As MASLD continues to grow globally, healthcare providers must stay proactive in identifying, screening, and managing patients at risk. Early diagnosis and intervention are the keys to reducing the global burden of this disease and preventing it from reaching advanced stages.
VLVbio are dedicated to providing cutting-edge, non-invasive diagnostic solutions that enable healthcare providers to detect MASLD in its early stages. As the prevalence of MASLD rises, early detection will become increasingly vital to addressing this public health challenge. Read more on how VLVbio’s M30 Apoptosense® ELISA can be incorporated into clinics to help identify patients here!
7 October 2024
Brochures
Keratin 18 in Alcohol-associated Liver Disease

- Keratin 18 in Alcohol-associated Liver Disease
- – What is ALD and what is the role of K18 in ALD?
- – How can measuring K18 aid in ALD patient management?
- – Learn about the use of K18 in early ALD diagnosis, detecting severe Alcoholic Hepatitis (AH), prognosis for survival, and predicting treatment response in ALD patient!
23 May 2024
Home Slider
The EASL Congress 2024

We are heading to Milan for the EASL Congress together with the European Association for the Study of the Liver!
We’re happy to yet again be exhibiting at the face-to-face #EASLcongress with EASL | The Home of Hepatology! We are happy to yet again be an industry partner for the biggest liver event in Europe!
The latest in hepatology is happening 📅 5–8 June.
🤝 Join us at the highlight of the year! Register today, and lets meet and discuss liver biomarkers at our booth.

For U.S. users: The VLVbio K18 assays have not been approved by the U.S. Food and Drug Administration
