News
15 November 2024
Blog
The Importance of Non-Invasive Testing in Early MASLD Diagnosis
As part of our ongoing effort to raise awareness about Metabolic Associated Steatotic Liver Disease (MASLD), we are exploring how advances in non-invasive testing (NITs) can transform early diagnosis. Early detection is crucial in preventing MASLD from progressing to advanced stages like fibrosis or cirrhosis, where treatment becomes more complex. NITs, such as serum biomarkers, imaging technologies, and elastography, are helping healthcare providers catch the disease before significant liver damage occurs.
Why Early Diagnosis Matters
MASLD often progresses without symptoms in its early stages, meaning patients can unknowingly live with the disease for years. By the time symptoms appear—often in the form of fatigue, abdominal swelling, or jaundice—patients may already have advanced fibrosis or cirrhosis. Early diagnosis is essential to allow healthcare providers to implement lifestyle interventions and treatment strategies that can slow or even reverse the disease.
Overview of Non-Invasive Testing Options
Traditionally, liver biopsies were the primary method for diagnosing liver diseases like MASLD. While still valuable in some cases, biopsies are invasive and may carry risks. The field has since evolved, and there are now several non-invasive methods available to assess liver health.
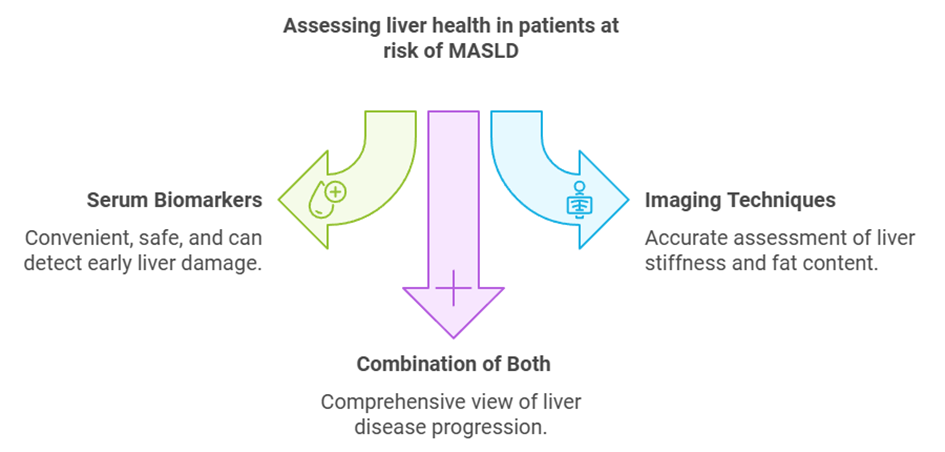
1. Serum Biomarkers
Serum biomarkers are among the most promising tools for detecting early liver disease. These tests measure specific proteins in the blood that are associated with liver damage, such as cell death (apoptosis) and fibrosis. Biomarkers, like the M30 Apoptosense® ELISA, help detect liver cell apoptosis, an early sign of disease progression. These tests are convenient, safe, and can be easily integrated into routine care for high-risk patients.
2. Imaging Techniques
Several imaging technologies are available to assess liver stiffness and fat content, helping clinicians evaluate liver health without invasive procedures. Elastography is commonly used to measure liver stiffness, while other imaging techniques like MRI-PDFF can quantify liver fat. These methods have significantly improved the accuracy of early diagnosis, although they may not always capture the underlying biochemical changes that serum biomarkers detect.
Both biomarkers and imaging tools offer valuable insights into liver health, and each has its place depending on the patient’s condition and risk factors. When used together, these technologies can provide a comprehensive view of liver disease progression.
How Non-Invasive Tests Improve Patient Outcomes
By utilizing non-invasive tests, healthcare providers can screen high-risk populations—such as individuals with obesity, Type 2 diabetes, or metabolic syndrome—more frequently and with less discomfort to the patient. This allows for:
Ongoing Monitoring: NITs allow clinicians to monitor disease progression and treatment effectiveness over time, adjusting interventions as needed.
Earlier Interventions: Once MASLD is diagnosed, patients can begin lifestyle changes (such as diet and exercise) or medical treatment earlier, improving the likelihood of reversing or halting the disease.
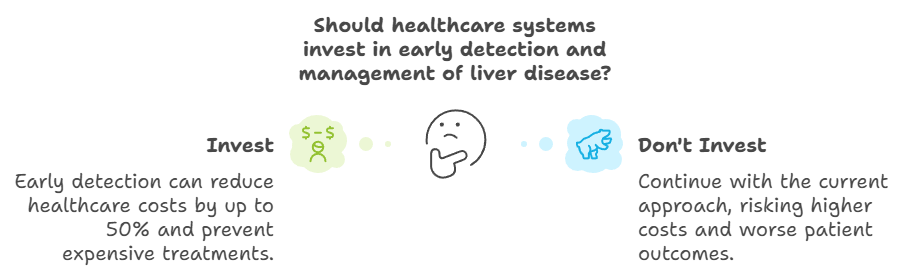
Cost-Effectiveness of Early Diagnosis
Early detection not only improves patient outcomes but also offers significant cost savings for healthcare systems. A study found that managing liver disease at earlier stages can reduce healthcare costs by up to 50% compared to treating advanced-stage complications such as cirrhosis or liver cancer (Younossi et al., Journal of Hepatology, 2020). In the U.S., the annual healthcare costs related to MASLD are estimated to exceed $100 billion, a number that continues to rise as MASLD prevalence increases (Younossi et al., Hepatology, 2020).
Research also highlights that patients with cirrhosis incur nearly 4.5 times higher medical costs than those diagnosed early. For example, annual direct medical costs for patients with cirrhosis are approximately $35,000, compared to $7,800 for those diagnosed at earlier stages (Tapper et al., Hepatology, 2020; Younossi et al., Journal of Hepatology, 2020). Early diagnosis can therefore prevent the need for expensive treatments such as hospitalizations and liver transplants, significantly reducing the economic burden on healthcare systems.
Conclusion
Non-invasive testing has opened new doors in the early diagnosis and management of MASLD. From serum biomarkers to imaging techniques, these tools are making it easier for healthcare professionals to diagnose liver disease before it progresses. By using these advanced diagnostics, clinicians can help prevent severe outcomes and improve patient quality of life. VLVbio is proud to support this shift toward earlier, more accurate detection through our non-invasive testing solutions. Read more here!
17 October 2024
Blog
Understanding MASLD and Its Growing Global Prevalence
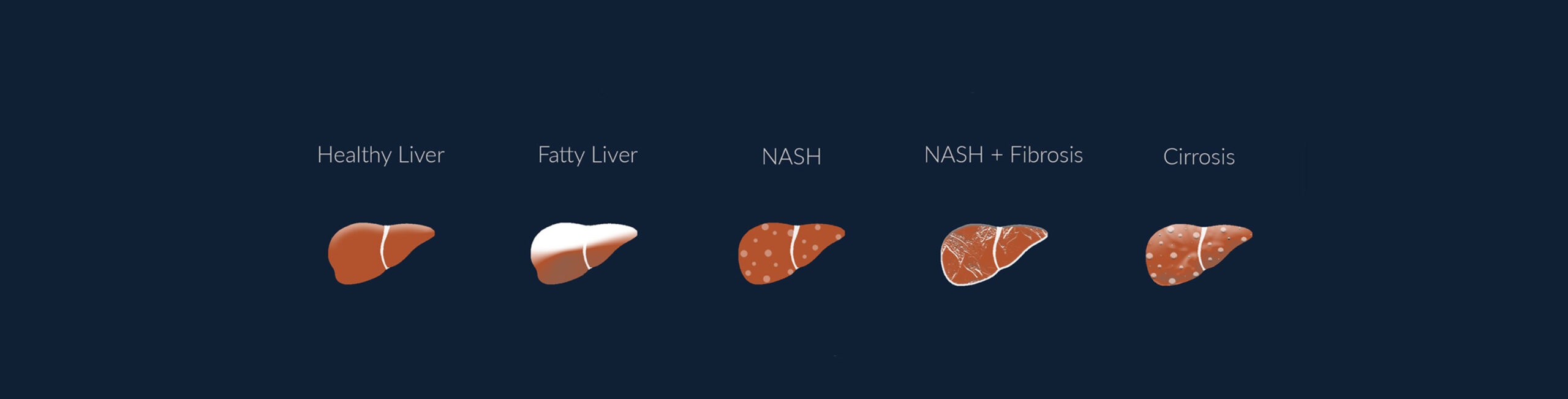
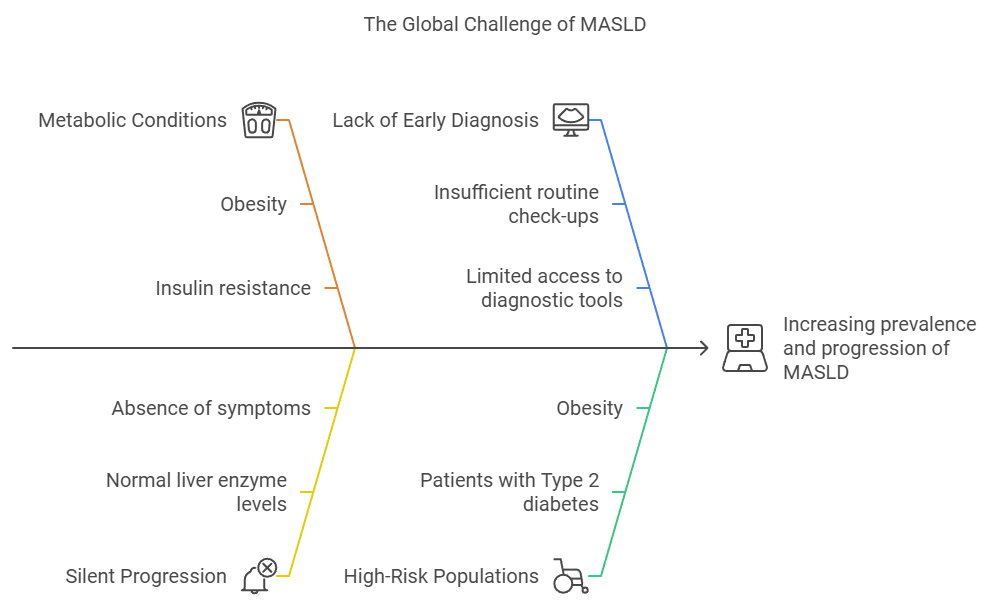
Metabolic Associated Steatotic Liver Disease (MASLD), also known as Non-Alcoholic Fatty Liver Disease (NAFLD), has emerged as a major global health challenge. Affecting roughly 30% of the world’s population, MASLD is closely tied to the rising rates of obesity, Type 2 diabetes, and metabolic syndrome. This condition, which can progress from simple liver fat accumulation to severe stages like MASH, fibrosis or cirrhosis, requires urgent attention from healthcare professionals to manage its growing prevalence.
What is MASLD?
MASLD is a spectrum of liver diseases where excess fat builds up in the liver without significant alcohol consumption. The condition ranges from simple steatosis (fatty liver) to more severe forms like metabolic dysfunction-associated steatohepatitis (MASH), liver fibrosis, and cirrhosis. The key risk factors driving MASLD are metabolic conditions such as obesity and insulin resistance.
The Growing Global Prevalence of MASLD
Over the past few decades, the prevalence of MASLD has steadily increased across the globe. Recent studies reveal that 30% of the world’s population is affected by MASLD, a significant rise from the 25% reported in 2016. In certain regions like Latin America and the Middle East, the prevalence rates exceed 40%, driven by the increasing rates of obesity and metabolic syndrome. By 2030, the number of MASLD cases is expected to surge, particularly in Southeast Asia, North America, and Western Europe, as the global burden of metabolic disorders continues to rise (The Global Epidemic of Metabolic Fatty Liver Disease, 2024).
This growing burden makes early detection more critical than ever. The silent progression of MASLD, particularly among high-risk groups such as those with Type 2 diabetes or obesity, often leads to late-stage liver disease by the time it’s detected. Early screening and intervention can prevent end-stage complications like cirrhosis and liver failure.
Why Early Diagnosis is Critical
Early diagnosis of MASLD is pivotal in preventing the disease from progressing to more severe stages, such as fibrosis, cirrhosis, or hepatocellular carcinoma (HCC). MASLD often develops silently, with patients experiencing few or no symptoms in the early stages. As a result, the disease can go undetected until it advances to a point where treatment options are limited, and the risk of irreversible liver damage increases.
Silent Progression to Severe Disease
Many patients with MASLD are unaware of their condition until it has progressed significantly. This is because MASLD often presents with normal liver enzyme levels, which may give a false sense of security during routine checkups. Without early intervention, MASLD can progress to MASH, a more severe form that involves liver inflammation and fibrosis. From there, the disease can further develop into cirrhosis or even liver cancer. The longer the disease goes undiagnosed, the fewer options remain for successful intervention. Detecting MASLD early provides a critical window for lifestyle changes and medical management that can reverse or slow its progression.
“I had no idea my liver was deteriorating for years. I felt fine until one day, my legs started swelling, and I felt extreme fatigue. By then, it was too late — my doctor told me I had cirrhosis, and my liver was severely damaged.” – MASLD patient with cirrhosis
Preventing Cirrhosis and Liver Cancer
Early diagnosis allows healthcare providers to implement interventions that can prevent patients from progressing to the advanced stages of liver disease. Patients with MASLD who are diagnosed early have a higher likelihood of responding to non-invasive treatments such as weight loss, dietary changes, and glucose control. Studies have shown that even a modest weight loss of 7-10% of body weight can significantly improve liver histology and prevent disease progression. When MASLD is detected at the stage of simple steatosis, the risk of developing cirrhosis or liver cancer is much lower than if the disease is allowed to progress without intervention.
In advanced stages like cirrhosis, the liver’s ability to function is severely compromised, and patients may require liver transplantation. Moreover, cirrhosis significantly increases the risk of developing hepatocellular carcinoma, a leading cause of cancer-related deaths worldwide. Therefore, detecting MASLD in its early stages can significantly reduce the incidence of both cirrhosis and liver cancer, which are often associated with poor prognoses.
Role of Non-Invasive Diagnostics
Recent advancements in non-invasive diagnostic tools have made early detection of MASLD easier and more accessible for clinicians. Tools such as imaging and serum biomarkers allow healthcare providers to assess liver fat and fibrosis without the need for invasive liver biopsies. This is particularly valuable in primary care settings, where regular screening of high-risk populations (such as those with obesity, diabetes, or metabolic syndrome) can be incorporated into routine check-ups.
These diagnostic tools also allow for risk stratification, enabling clinicians to categorize patients based on their likelihood of progressing to advanced liver disease. This helps in tailoring interventions and deciding when to refer patients to specialists for more advanced care, reducing unnecessary referrals and allowing for more efficient healthcare resource allocation.
Managing High-Risk Populations
Patients with Type 2 diabetes, obesity, and other metabolic disorders are at particularly high risk for MASLD. In fact, up to 70% of people with Type 2 diabetes and 90% of obese individuals have MASLD. Early diagnosis in these populations is crucial, as they are more likely to progress to severe liver disease. By integrating liver health assessments into regular diabetes or obesity management plans, healthcare providers can catch MASLD before it progresses, allowing for earlier lifestyle modifications and potentially reducing the burden of advanced liver disease in these high-risk groups.
Improving Patient Outcomes and Reducing Costs
Early diagnosis and intervention not only improve patient outcomes but also reduce healthcare costs in the long run. Treating MASLD in its early stages is far less costly than managing advanced liver disease, which often requires hospitalizations, expensive treatments, or even liver transplantation. Moreover, patients diagnosed early are more likely to adhere to preventative measures such as diet and exercise, which can greatly improve liver health and reduce the need for long-term medical intervention.
Conclusion
As MASLD continues to grow globally, healthcare providers must stay proactive in identifying, screening, and managing patients at risk. Early diagnosis and intervention are the keys to reducing the global burden of this disease and preventing it from reaching advanced stages.
VLVbio are dedicated to providing cutting-edge, non-invasive diagnostic solutions that enable healthcare providers to detect MASLD in its early stages. As the prevalence of MASLD rises, early detection will become increasingly vital to addressing this public health challenge. Read more on how VLVbio’s M30 Apoptosense® ELISA can be incorporated into clinics to help identify patients here!
7 October 2024
Brochures
Keratin 18 in Alcohol-associated Liver Disease

- Keratin 18 in Alcohol-associated Liver Disease
- – What is ALD and what is the role of K18 in ALD?
- – How can measuring K18 aid in ALD patient management?
- – Learn about the use of K18 in early ALD diagnosis, detecting severe Alcoholic Hepatitis (AH), prognosis for survival, and predicting treatment response in ALD patient!
23 May 2024
Home Slider
The EASL Congress 2024

We are heading to Milan for the EASL Congress together with the European Association for the Study of the Liver!
We’re happy to yet again be exhibiting at the face-to-face #EASLcongress with EASL | The Home of Hepatology! We are happy to yet again be an industry partner for the biggest liver event in Europe!
The latest in hepatology is happening 📅 5–8 June.
🤝 Join us at the highlight of the year! Register today, and lets meet and discuss liver biomarkers at our booth.
17 April 2024
Home Slider
K18 – the Key to Unlocking Informed Decisions in MASH Drug Trials
Join us for an enlightening webinar presented by Jessica Tuohy, a distinguished partner and colleague from the US, focusing on the significance of K18 as a biomarker in the development of treatments for MASH (Metabolic Associated Steatohepatitis). This webinar is essential for drug developers, doctors, researchers, and key opinion leaders in the field of metabolic liver diseases, offering deep insights and key research findings.
Event Details:
- Date: Tuesday, April 23
- Time: [1pm EDT, 7pm CET]
Webinar Overview: This session will cover several critical areas:
- – An Introduction to the Growing Epidemic of MASLD (Metabolic Associated Steatotic Liver Disease)
- – Challenges in Developing Therapies for the Treatment of MASH
- – An Overview of K18 and Its Role in MASH Pathogenesis
- – A Review of Data That Support the Value of K18 as a Biomarker for Assessing the Efficacy of Drug Candidates in MASH Clinical Trials
Who Should Attend:
- – Drug developers in the field of MASLD and MASH.
- – Physicians and healthcare professionals specializing in hepatology, gastroenterology, and related fields.
- – Medical researchers focusing on liver disease and drug development.
- – Key opinion leaders in metabolic liver diseases.
Registration: Please register here to secure your spot in this informative webinar. Registration is required to access this online event.
Additional Information: If you are unable to attend the live event, a recording will be made available to all registered participants, ensuring you can access this webinar at your convenience.
We look forward to welcoming you to what promises to be an informative and transformative session.
For more information, please contact us at marketing@vlvbio.com.
Don’t miss out on the chance to advance your knowledge in MASH treatment and diagnostics. Reserve your spot today!
4 March 2024
Blog
World Obesity Day
Today marks World Obesity Day, a global initiative aimed at raising awareness about the growing epidemic of obesity and its profound impact on health. While the visible effects of excess weight are well-known, it’s crucial to delve deeper into the associated health risks, such as Metabolically Associated Fatty Liver Disease (MASLD), a silent threat that often goes unnoticed until it reaches critical stages.
The Silent Threat: MASLD
MASLD, a condition linked to obesity, is a silent liver disease that often progresses without any overt symptoms. As the world grapples with rising obesity rates, MASLD has become a significant concern due to its subtle nature and potential to lead to severe liver damage. The liver, a vital organ responsible for various metabolic functions, can be adversely affected by the accumulation of fat, inflammation, and fibrosis associated with MASLD.
Raising Awareness: A Necessity for Change
This World Obesity Day, it’s imperative to shed light on MASLD, a condition that lurks beneath the surface, silently compromising one’s liver health. Awareness campaigns and educational initiatives play a pivotal role in encouraging individuals to make informed lifestyle choices, promoting early detection, and seeking medical advice.
Diagnostics and Treatments
In the fight against MASLD, advancements in diagnostic tools offer a glimmer of hope. Cutting-edge technologies, such as non-invasive biomarkers and imaging techniques, enable healthcare professionals to detect MASLD in its early stages, allowing for timely intervention and before last stage disease. Regular screenings for individuals at risk can make a substantial difference in preventing the progression of this silent liver disease.
As we recognize World Obesity Day, it’s essential to emphasize the need for innovative treatments for MASLD. Pharma companies and researchers are exploring novel therapeutic approaches, ranging from lifestyle interventions to pharmaceutical developments, aiming to mitigate the impact of MASLD on liver health. Collaborative efforts between healthcare providers, researchers, and policymakers are vital to develop effective strategies for preventing and managing MASLD in the context of the global obesity crisis.
Addressing the obesity epidemic and its associated health issues, including MASLD, requires a collective effort. Governments, healthcare professionals, community leaders, and individuals alike must collaborate to implement policies that promote healthy living, foster early detection, and ensure access to appropriate treatments.
On this World Obesity Day, let us come together to raise awareness, destigmatize health issues related to obesity, and advocate for the development of comprehensive strategies that address not only the visible consequences but also the silent threats like MASLD. By working together, we can pave the way for a healthier and more informed future.
Read more on how the M30 Apoptosense® ELISA can aid in diagnosing NASH and fibrotic NASH
Read more on how the M30 Apoptosense® ELISA can aid in diagnosing pediatric NASH
Read more on how the K18 assays can help in NAFLD clinical trials
Reda Elkhatib
