News
15 August 2023
Home Slider
A Message From the VLVbio Team
Hello dear friends and supporters,
As the warmth of the Swedish summer slowly gives way to the soon coming colorful embrace of autumn, we find ourselves reenergized and ready to continue our mission. We are delighted to be back and to share with you the passion that fuels our purpose.
After a well-deserved break, we’re excited to return to our work, more determined than ever to raise awareness about chronic liver conditions and make strides towards our vision of a world with no undiagnosed liver diseases. The sunshine and relaxation (and quite a lot of rain!) of the summer months have provided us with the inspiration needed to push forward and make a lasting impact on the lives of those affected by these silent ailments.
The Swedish summer has always been a time of reflection, growth, and rejuvenation for us. We’ve taken this opportunity to recharge our energies, connect with our loved ones, and find fresh perspectives to bring back to our mission.
We extend our heartfelt gratitude to all of our partners and friends for this half of the year. Together, we shall continue our work toward making a difference in the lives of countless liver disease patients, with raised awareness, early detection, and well established treatment and follow-up.
As we dive back into our work, we invite you to join us on this journey towards our vision. Keep an eye out for our upcoming events, publications, and educational initiatives during the fall and winter.
Thank you for being a part of our community, for your trust, and for your commitment. Together, we can make a significant impact and work towards a world where no one has to suffer from undetected liver conditions. Here’s to a season of renewed purpose and the promise of a healthier, brighter future for all. Please enjoy some lovely pictures from a typical summer here in Stockholm, Sweden.
With gratitude and determination,
The VLVbio Team





26 May 2023
Home Slider
The EASL Congress 2023

We are heading to Vienna for the EASL Congress together with the European Association for the Study of the Liver!
We’re happy to yet again be exhibiting at the face-to-face #EASLcongress with EASL | The Home of Hepatology! We are happy to yet again be an industry partner for the biggest liver event in Europe!
The latest in hepatology is happening 📅 21–24 June.
🤝 Join us at the highlight of the year! Register today, and lets meet and discuss liver biomarkers at our booth.
18 May 2022
Brochures
Combining M30 Apoptosense® ELISA with FIB-4
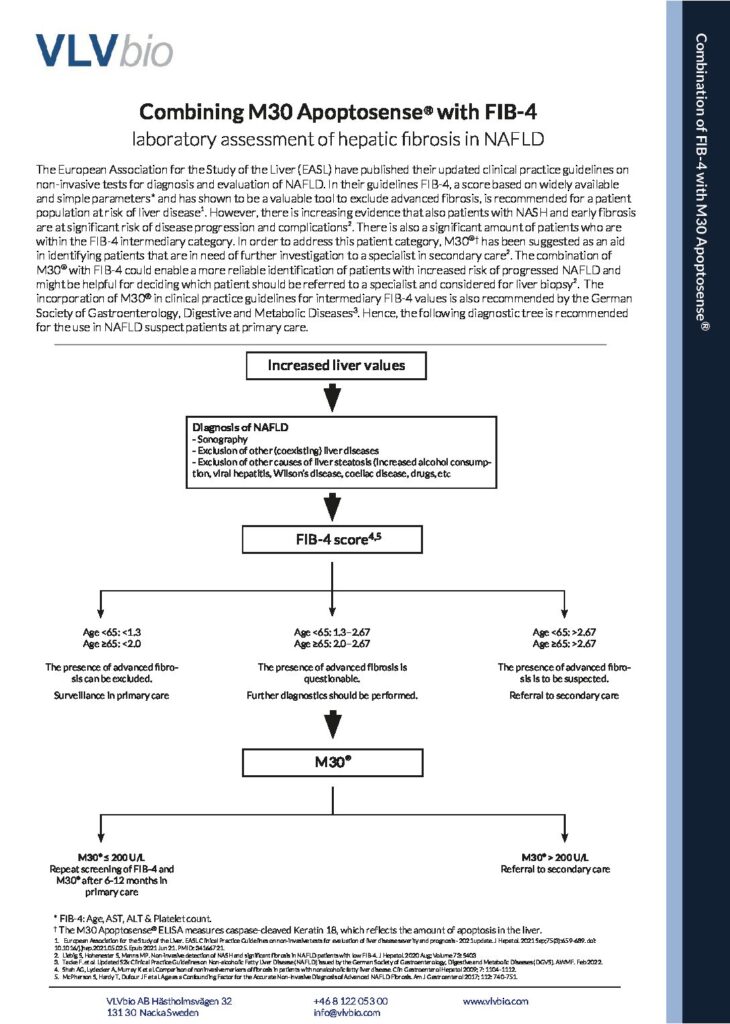
Learn how to combine the use of FIB-4 together with the M30 Apoptosense® ELISA to asses hepatic fibrosis in NAFLD already at primary care!
As NAFLD global prevalence is at a record high, and continuing to increase, there is a great need to non-invasively assess patients at risk of developing NASH and fibrosis already at primary care.
By using Non-Invasive Tests to evaluate risk group patients already at primary care, only the patients at highest risk of disease progression and liver related complications will proceed to a specialist in secondary care for further investigation.
FIB-4 has shown to be a valuable tool to exclude advanced fibrosis, and is widely recommended in clinical guidelines to be used. The combination of the M30 Apoptosense® ELISA with FIB-4 could enable a more reliable identification of patients with increased risk of progressed NAFLD and might be helpful for deciding which patient should be referred to a specialist and considered for liver biopsy.
Hence, in accordance with the German Society of Gastroenterology, Digestive and Metabolic Diseases Guidelines, a diagnostic tree incorporating FIB-4 and M30® is recommended for the use in NAFLD suspect patients at primary care.
27 April 2022
Brochures
Utility of Caspase-Cleaved Keratin 18 to Diagnose NASH in Patients with Obesity
NASH and all its complications are especially prevalent in patients with severe obesity and type 2 diabetes. Yet few study data exist on biomarker performance within this patient group, in order to move away from the necessity of liver biopsy in clinical practice.
Sami Qadri, MD & Hannele Yki-Järvinen, MD et al. performed a study to evaluate the use of the M30 Apoptosense ELISA® to diagnose NASH or fibrotic NASH in an obese cohort.
They found that plasma levels of M30® associated with the full histological spectrum of NASH and also had a good ability to identify both NASH and fibrotic NASH with high sensitivity and specificity!
9 May 2022
News
The International Liver Congress 2022
🚌 We’re aboard the bus to London for a face-to-face ILC2022 with EASL | The Home of Hepatology! We are happy to yet again be an industry partner for the biggest liver event in Europe!
The latest in hepatology is happening 📅 22–26 June.
🤝 Join us at the highlight of the year! Register today, and lets meet and discuss liver biomarkers at our booth.




