News
9 September 2020
News
PEVIVA® Keratin 18 biomarkers and COVID-19
The coronavirus-2 (SARS-CoV-2) is currently a public health crisis all over the world with a fatal rate between 2-6 %, and mostly affecting our elderly and people with underlying risk factors such as diabetes, heart disease and hypertension. New research shows that hepatic dysfunction has been seen in 14-53 % of the covid-19 patients, and acute liver injury is associated with higher mortality. The liver injury in covid-19 patients could be a result of the changes in the host cells caused by the viral invasion (cytopathic effect), sepsis or drug-induced liver injury1.
M30 Apoptosense® ELISA and M65 EpiDeath® ELISA are prognostic biomarkers at ICU admission and for drug-induced liver injury progression
For research use only in USA and Canada.
M30 Apoptosense® ELISA is correlated with biomarkers of inflammation, renal- and liver failure in critically ill patients. A study performed by Koch et al., demonstrates that patients at the ICU that later died had higher levels of serum M30® at admission in comparison to patients that survived. Patients with M30® values >250,8 U/L displayed an excessive short-term mortality2.
The PEVIVA® Keratin 18 biomarkers have also been recommended for assessing and anticipating the risk of progression in severe drug-induced liver injury patients in clinical trials, and the biomarkers are strongly associated with liver related death or transplantation within six months of drug-induced liver injury onset3.
The PEVIVA® Keratin 18 biomarkers are the ideal tools for disease monitoring and prognosis in research- and clinical use in critically ill covid-19 patients.
Covid-19 IgM/IgG Rapid Tests
During the current covid-19 pandemic, a lot of focus and energy has been put into testing people for the new corona virus and thus tracking and managing the spread of infection in society. VLVbio has therefore chosen to include a rapid test for Covid-19 IgM and IgG antibodies in our product portfolio. The antibody test we offer is a POC test that within 10 minutes gives a reliable answer if the patient has IgM and / or IgG antibodies to the new coronavirus. The test has been studied extensively in previous research, including a study where the Danish Statens Serum Institut (SSI), examined and validated the test4. The rapid test has been shown to have a high sensitivity of up to 96% and a specificity of up to 100% from various studies performed on a total of 857 patients, making it one of the best performing tests available today. The test also does not show any cross-reactivity with other common viruses.
The test, 2019-nCoV IgM / IgG Rapid Test, is imported from the Swiss international distributor TECOmedical AG, where development and production takes place at Dynamiker Biotechnology Co. together with the Chinese Institute of Medical Biology.
The test is of course CE-IVD marked, and has been shown to have similar or even more sensitive test performance than the antibody tests used in the clinical labs for covid-19 today.
Please contact us for more references and quotation.
References
- Jothimani et al, 2020, COVID-19 and the liver. DOI: https://doi.org/10.1016/j.jhep.2020.06.006
- Koch et al, High circulating caspase-cleaved keratin 18 fragments (M30) indicate short-term mortality in critically ill patients. DOI: https://doi.org/10.1155/2018/8583121
- The Drug induced liver injury work package of Innovative Medicines Initiative SAFE-T Consortium and The Hepatotoxicity Working Group of Critical Path Institutes PSTC.
- Lassaunière et al, Evaluation of nine commercial SARS-CoV-2 immunoassays. DOI: https://doi.org/10.1101/2020.04.09.20056325
12 June 2020
News
International NASH day 2020 – Chat Interview
To celebrate this years International NASH day we are proud to have interviewed Dimitar Tonev through live chat! Dimitar has a large and rich background within liver diseases and is one of the experts in the NASH field. Find out more about the disease, at risk patients, diagnosis, treatment options and much more to raise awareness on NASH!


•Reda Elkhatib 15:05
Hi Dimitar, how are you?
•Dimitar Tonev 15:05
I’m fine, thanks How about you?
•Reda Elkhatib 15:06
I am fine, thanks!Thank you for doing this chat interview with us, to try to raise awareness on NASH during The International NASH day 2020.
•Dimitar Tonev 15:07
Thats a great idea I am privileged to support today.
•Reda Elkhatib 15:09
To start off, I must say you are one of the experts in the NASH field. Could you please describe your experience within NASH and liver diseases?
•Dimitar Tonev 15:09
Sure
I have been involved extensivelly with the clinical development and later market utilisation of new treatments for HBV and HCV, ever since the times we were using conventional interferon. In the last 6-7 years I have refocused my attention to development of agents for the treatment of NASH and smaller liver diseases like PBC and PSC.
I was one of the first medical directors of Intercept (maker of Ocaliva) in Europe and later consulted many small biotech companies on their developmental programs in NASH space, including here diagnostic technologies, devices and even big clinical research organisations like Iqvia.
•Reda Elkhatib 15:14
That’s an impressive career I must say, congratulations and thank you for all the work you have been doing!
•Dimitar Tonev 15:14
Thank you, Reda
•Reda Elkhatib 15:14
To get into it, as you know, NAFLD affects around 25% of the global population and many of these patients develop NASH. Could you describe the health and economic burden on society this disease brings forth?
•Dimitar Tonev 15:14
I agree
recently NASH became the number one reason for a liver transplantation almost everywhere in the developed world, taking this worrisome crown from HCV. Associated mortality and consumption of health care resources is unparalleled in the history of liver diseases.
Its still a subject of debate but NASH probably has a bimodal connection with T2DM-meaning that diabetes drives NASH further and NASH is making diabetes worse.
•Reda Elkhatib 15:18
That really is worrisome!
•Dimitar Tonev 15:18
I know, right?
•Reda Elkhatib 15:19
Even though many patients are affected, and as you say NASH is widely associated with T2DM, there are still many that do not know of the disease. Why do you think that is?
•Dimitar Tonev 15:19
Yes
On one hand that’s the typical issue with other asymptomatic diseases we frequently typify as “iceberg structure”. On the other it’s perhaps due to the fact that the majority of cases are a responsibility of diabetologist and GPs which are rarely focused on the liver health. Minority of these patients develop advanced fibrosis and cirrhosis and are being referred to hepatologists and gastroenterologists.So physicians that see these patients every day are rarely equipped with the tools and know how to recognise an early and perfectly treatable liver disease.
•Reda Elkhatib 15:23
That is really interesting..How should patients know they are at risk and can these patients be diagnosed in any way?
•Dimitar Tonev 15:28
I would suggest our more and better aware patients with risk factors (males, beyond 60, with obesity and T2DM) to start requiring simple functional liver tests as ALT, AST to be done at their regular follow up sessions with their health provider. These tests are very cheap and easily available everywhere. Given their title specificity, there is a need for a secondary (sometimes called sequential or trigger) more specific biochemical test to be done as well as abdominal sonography (echo) or transient elastography (fibroscan) as a confirmatory methodology.
•Reda Elkhatib 15:30
Those are some good points. A livery biopsy is considered the golden standard by many to determine the diagnosis. Is that still the case, and which patients should go through that procedure?
•Dimitar Tonev 15:32
Yes and no. Currently liver biopsy is not widely recommended for patients in normal clinical practice because the results (so called histology reading) would not change the way these patients are being treated.
As a specific case, if there is a chance and interest on a patients side to be included in advanced Clinica trials (we call those phase IIb and phase III) he or she will have to agree to have one or more liver biopsies. For the time being regulatory drug agencies do all require this histological assessment to be the primary endpoint of all registration studies for potential anti NASH medications.Liver biopsy is a rich and classical source of information about the structure of liver tissue but it’s not without shortcomings and risks.It has been already successfully replaced by other diagnostic modalities in the successful drug development and treatment of diseases like HBV, HCV and PBC.
•Reda Elkhatib 15:37
Certainly, and considering the millions of patients that would require testing, performing a liver biopsy on everyone is not feasible at all!
•Dimitar Tonev 15:38
Exactly
we don’t have the technical ability to do that sophisticated and expensive test to everybody.
•Reda Elkhatib 15:39
Yes, I agree. Especially not on a global scale!
•Dimitar Tonev 15:39
Exactly
•Reda Elkhatib 15:40
What would current treatment options for NASH patients be, since there is no drug available today?
•Dimitar Tonev 15:43
There is currently anecdotal data to support “treatment” with traditional dietary restrictions and physical activity. If we are successful with that and decrease patients body weight with app 10% the liver status will almost completely normalise. The majority of patients would not be able to follow such recommendations for a long enough period of time. They are left with the potential benefits of some diabetic and anti-obesity medications that are currently registered and even reimbursed for these concomitant (coexisting) diseases. FDA will soon discus the potential provisional licence for the first successful Anti NASH medication called obeticholic acid (Ocaliva)
.•Reda Elkhatib 15:45
Yes, excercise and diet are usually treatment programs that do not get followed and that’s a big problem.
•Dimitar Tonev 15:46
Definitely its not easy break the pattern of little physical activity and extra caloric food we are all subjected to…
•Reda Elkhatib 15:46
Do you think it is still necessary to diagnose patients already today, even though no drug is available?
•Dimitar Tonev 15:48
I believe so. Patients with diagnosed NASH (both non-invasive and histological sense) will be ready for clinical trials and new agents when they became available in their area/country. Sometimes extreme obesity could be successfully treated with surgical interventions as balloons and bariatric techniques -some countries even reimburse this fully for patients with BMI>35 or 40.
•Reda Elkhatib 15:49
That is a good point, and I must say that I agree!To conclude, we of course must talk about the current pandemic that has shaken the world. Do you think the Covid-19 outbreak will have any effect on NAFLD and NASH prevalence? Since many people are quarantined with limited access to exercise and a healthy diet.
•Dimitar Tonev 15:50
I’m not sure we see anecdotal increase of folks exercising in parks and fields after lockdown.
•Reda Elkhatib 15:52
That is interesting, I didn’t know that!
•Dimitar Tonev 15:53
On a serious note NAFLD is perhaps an additional risk factor to get serious complications from covid-19 infection. In a recent article patients with NAFLD were 6 times more likely to get complications even if you adjust (compensate) for the underlying diabetes and obesity which are independent risk factors in their own right.The viral environment and associated public believes definitely do delay access to specialists, clinical trials or any other treatments in many cases.
•Reda Elkhatib 15:55
That is really worrisome. The liver has been discussed in research also as one of the affected organs of the virus.
•Dimitar Tonev 15:56
I agree Viral infection does activate certain typical for the liver tests which makes it difficult to control disease activity or the effects of your treatments.
•Reda Elkhatib 15:57
Yes, that is troubling. Let’s hope to see a successfull vaccine developed soon!
•Dimitar Tonev 15:58
I look forward to it as well as more investment in the somehow forgotten Virology drugs.
•Reda Elkhatib 15:59
Yes, I agree
Dimitar, thank you very much for your time and replies, it was a pleasure writing with you and much appreciated!!
•Dimitar Tonev 15:59
Likewise all the best with the rest of this important series of interviews!
•Reda Elkhatib 16:00 Thank you very much, we must all work together to try to raise NASH awareness!
Abbreviations Used
ALT: Alanine Aminotransferase
AST: Aspartate Aminotransferase
FDA: U.S. Food and Drug Administration
GP: General Practitioner
HBV: Hepatitis B Virus
HCV: Hepatitis C Virus
NAFLD: Non-alcoholic Fatty Liver Disease
NASH: Non-alcoholic Steatohepatitis
PBC: Primary Biliary Cholangitis
PSC: Primary Sclerosing Cholangitis
T2DM: Type 2 Diabetes Mellitus
30 April 2020
News
Covid-19
Company policy during the covid-19 pandemic
Stockholm, Sweden, March 13th 2020
As the covid-19 crisis is further expanding, VLVbio is deeply committed to help fighting against the pandemic.
As a Swedish company whose mission is to assess and manage human health and to protect people against disease, we are now extending our mission to protect our employees, customers and partners against covid-19.
Therefore:
- According to recommendations from the Swedish Goverment, the majority of the staff is operating from home.
- Interactions with professionals, customers and partners are replaced with digital solutions.
- Products are distributed as usual, with a possibly prolonged delivery time due to temporary policies of the Freight forwarders.
We remain committed to continue our activities the best we can and to support our customers with great spirit and engagement.
19 December 2019
News
Coming soon: M65 EpiRat™ ELISA
The latest addition to the PEVIVA product family will be released soon!
M65 EpiRat™ ELISA
The new edition to the PEVIVA product line, M65 EpiRat™ ELISA, is optimized as a rat specific assay and represents the final piece of the puzzle for the complete K18 portfolio, making the PEVIVA product line available and valuable in all stages of drug development.
Figure 1. PEVIVA product line in Drug Development
With the new arrival of the M65 EpiRat™ ELISA, the PEVIVA product line is now available throughout all stages of drug development, from the discovery and pre-clinical phase up until the clinical application of the drug.
Technique
The M65 EpiRat™ ELISA is based on the M5 and M6 antibodies, which bind to the biomarker Keratin-18 (K18). The concentration of K18 reflects the amount of overall cell death, both apoptosis and necrosis, in K18 positive cells. Levels of K18 are commonly elevated when the liver endures damage, through disease or toxicological effects.
Toxicology
The M65 EpiRat™ ELISA can be used in toxicological studies. As rodent species are commonly used globally in traditional toxicology testing guidelines to predict human health toxicity outcomes, the M65 EpiRat™ will help by quantifying the amount of cell death in hepatocytes to assess liver toxicity.
Research
In pre-clinical studies, the M65 EpiRat™ ELISA can be used to analyse the effect of drugs on treatment effect and disease progression for example for new compounds in Non Alcoholic Fatty Liver (NAFL) and Non Alcoholic Steatohepatitis (NASH).
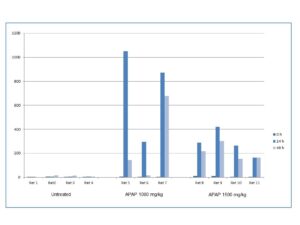
Figure 2.
11 Plasma samples from untreated rats and rats treated with different Paracetamol (APAP) doses were run using the M65 EpiRat™ ELISA. The figure shows an increase in M65 EpiRat™ values in rats treated with 1000 mg/kg APAP and 1500 mg/kg APAP, in comparison to the untreated rats. Blood was drawn after 0, 24 and 48 hours for each rat.

M65 EpiRat™ ELISA’s unique characteristics:
- Rat specific measurement of total cell death in K18 positive cells
- Quantification of K18 in rat serum and plasma samples.
- Convenient ready to use format and can be slit up for use at several occasions
We are very happy to welcome the M65 EpiRat™ ELISA as the newest member of the PEVIVA product line!

12 June 2019
News
June 12, 2019 – The International NASH day
Today we celebrate the international NASH day in order to together raise awareness in the fight against the NASH epidemic!
Read the blog post on how we should shed light on the disease and raise global awareness.
Today is International NASH Day! VLVbio is proud to join the Global Liver Institute (GLI) in raising awareness of this important health issue.
Non-alcoholic steatohepatitis, or NASH, has been called an epidemic, a ticking time bomb, and a silent tsunami. It is the progressive form of non-alcoholic fatty liver disease (NAFLD), and affects more than 115 million people worldwide. An estimated 357 million people will be affected by 2030.
Because NASH symptoms are not overt, NASH is often underdiagnosed and underreported. NAFLD and NASH are major risk factors for other health conditions: more than 70% of patients are obese, up to 75% have type 2 diabetes, and anywhere from 20-80% have hyperlipidemia. Unchecked, NASH may lead to cirrhosis, liver cancer, and liver transplant.
Despite its prevalence and serious health consequences, NASH is relatively unknown. In a recent survey, only 6% of at-risk individuals had ever heard of NASH.
In order to raise awareness, the first International NASH Day was launched on June 12th last year. Now the second annual International NASH day is set to build off last year’s success and further spread the word about NASH and its effects.

“Our goal is to shine light on this silent epidemic.” said Donna Cryer, GLI’s President and Founder.
The golden standard for diagnosing NASH today is through biopsy which is an invasive procedure which comes with many patient burdens and high costs. One of the pressing needs in the field is to implement non-invasive and low-cost techniques for diagnosing and staging the disease. One of the discussed serum markers is Keratin 18 (K18), which is leaked out of liver cells after cell death. Cell death is understood to be one of the contributing factors for disease progression in NASH. By measuring caspase-cleaved K18 (ccK18) and total K18 through the M30® and M65® antibody assays, you can quantify the amount of apoptosis and total cell death in the liver which helps in NASH diagnosis.
“We at VLVbio want to spread information on the disease as well as increase the acceptance and adoption of non-invasive diagnostic techniques.” says Slavica Brnjic, CEO of VLVbio.
For more information, visit globalliver.org/IND
/ Reda El Khatib, MMSc, Marketing Specialist, VLVbio
